Modernizing EDC With AI
Veridix is modernizing EDC by incorporating AI and automation into the core processes used to ingest, monitor, and correct the critical data captured for your clinical trial. Many aspects of data management benefit from AI and automation including:
AI-based capabilities for importing non-EDC data sources
Automated builds and edit check based on AI recommendations
Data correction recommendations at point of entry
Monitoring to automatically detect and correct site-based entries
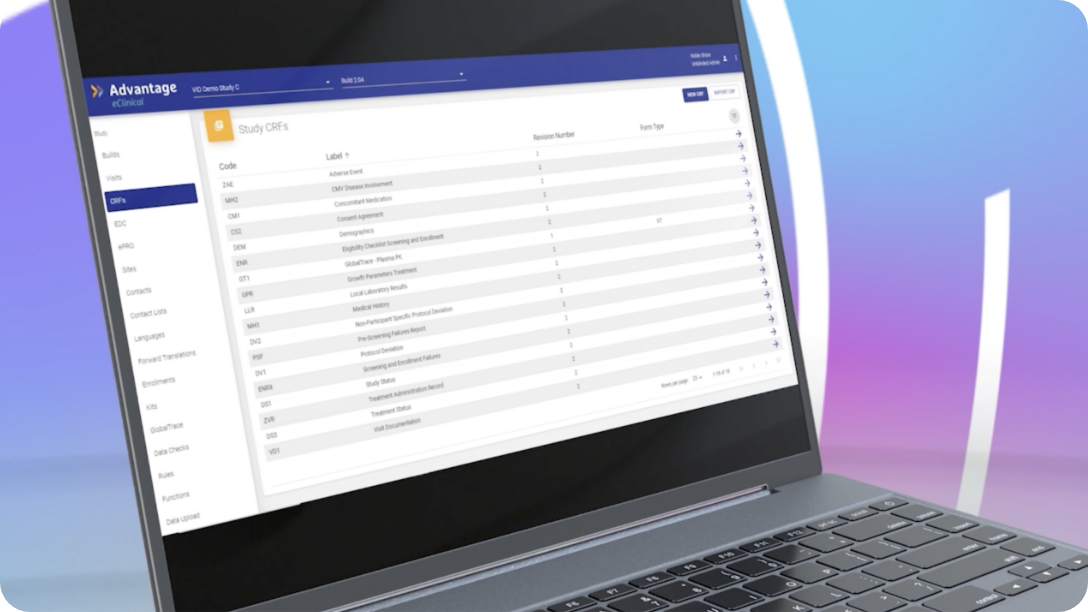

Direct Data Capture
Our Electronic Data Capture platform enables direct data capture capabilities with the ability to securely attach any file or document.

Coding
Integrated coding module for verbatim terms to the MedDRA and WHODrug dictionaries, including automated and manual coding across multiple dictionary levels, review and reclassification of coding decisions, and support for dictionary upcoding.
Single Sign-On (SSO)
Users can log in with a single account to access any Veridix eClinical Cloud application, connected apps, or websites.
Faster Launch, Faster Changes
One place for all build activities eliminates redundancy and minimizes error. Build a study once and the configuration is automatically applied to any Advantage eClinical Cloud application. An easy-to-use publishing tool allows mid-study changes and amendments to be deployed quickly without any system downtime.
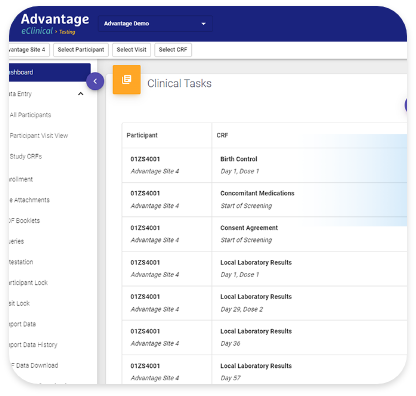
Quick & Easy Remote Source Data Verification (SDV)
CRAs can remotely verify site-entered data are consistent with source documents (or other electronic systems), can indicate discrepancies at the field level, and track them until resolution. Automated resolution of discrepancy queries saves time by eliminating re-review.
Real-time Visibility & Access to Data
Visually track enrollment progress, query, and performance KPIs across sites. Visualizations make it easy to identify site compliance issues. Access pre-built interactive reports, charts, and data aggregations or build your own.

Latest Resources
Fact Sheet
EDC Factsheet
Case Study

Less Than 1 Month to Unlock Database
and Capture 2,700 Lab Records.