Advantage eClinical supports a BYOD model and offers a flexible solution which is seamlessly integrated within a unified platform. Launch studies quickly and with confidence and provide study participants with a simple, intuitive interface that facilitates a positive, patient-centric experience.
Book a Demo
Learn more about our cloud-based suite of applications for study design, data capture, randomization and reporting.
Flexible and Configurable
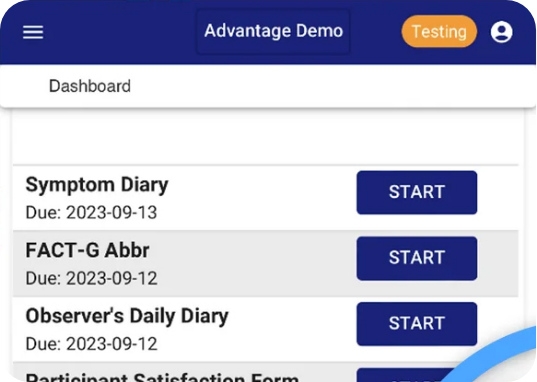
Configurations to fit the needs of any study – supports a number of validated instruments, offers flexibility form displays and study-level configurations for form access and behavior.
Mobile-Centric Design
With a modern, responsive UX, patients can access the system using common web browsers on any tablet or smartphone, including their own device (BYOD).
Easy Access, Anywhere
One-click access via email or SMS reminders and patient self-serve PIN reset increases access and ease of use. Localization support allows global participants to submit data in their native language.


Improved Compliance
and Engagement
Maintain high levels of patient compliance and retention with configurable automated emails and SMS reminders. Monitor compliance in real time via the platform or included reports.

Saves Time and
Reduces Errors
Using Veridix eClinical eCOA limits data transcription errors with real-time data visibility, saves study staff significant time in data entry and enables follow-up on AE alerts.

Seamless and Unified
Veridix eClinical eCOA is fully integrated with Veridix EDC as one application, providing sites with a simplified experience and real time unified data access for patient management and compliance monitoring. It enables sponsors / CROs to deploy EDC and eCOA/ePRO in a single environment, eliminating the burden on managing multiple solutions and data integration.
ECOA
Direct data capture from clinician and patients on any web-enabled devices – learn more here.
Book a Demo
Discover how our Direct Data Capture can streamline processes and accelerate your clinical trials.