Real-time & Purpose-built
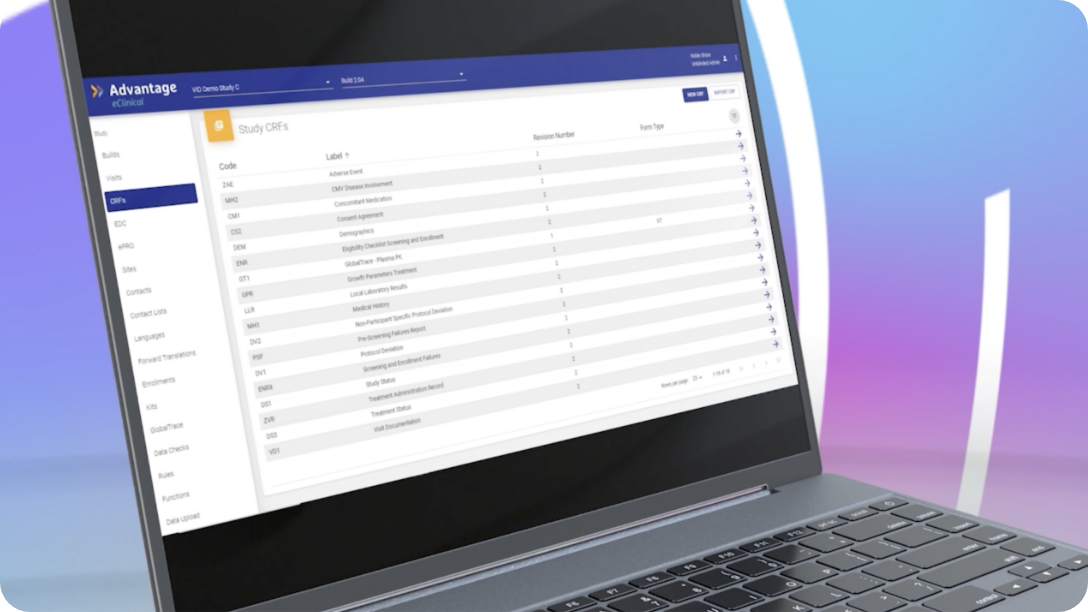
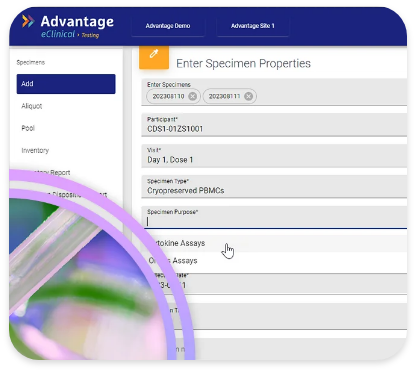
GlobalTrace is purpose-built to track every step of the specimen’s journey in real-time when sample integrity and chain of custody are critical. The information recorded includes, specimen attributes, linkage to clinical data, current & previous locations, shipment workflow, issue management. GlobalTrace can track aliquoted/pooled specimens, maintaining the linkage to originals.
Efficient Operations
GlobalTrace simplifies and automates tasks performed by all of the specimen processors. Tracking of residual volume and consent for future use makes it possible for sponsors to know whether specimens are available for testing under current protocol(s) or to plan for future use studies.
Central Inventory
GlobalTrace does not replace the specimen inventory maintained by clinical sites, specialty labs, and/or specimen repositories. Instead, it maintains a central inventory which shows which of these entities currently holds the specimen, as well as the full history of transfers and use.
Easier Assay Management
GlobalTrace facilitates the exchange of blinded samples between labs, facilitating assay development, validation, or qualification and/or calibration of one assay against another

Reduces Errors & Omissions
Proven to reduce errors or omissions in specimen collection, lost, misidentified, or ruined specimens, discrepancies between clinical and laboratory inventories, errors or omissions in laboratory results reporting, and lack of compliance with participant consent for future use.

Real-time Data & Visibility
Complete and real-time integration of clinical and specimen data makes it easier to select picklists of specimens for testing, enabling efficient study conduct where only a subset of specimens is tested or where staged/batched testing occurs.
Seamless & Unified
GlobalTrace, unified in the Veridix eClinical Cloud platform, facilitates easy linkage between specimen and clinical study data for analysis and reporting purposes.
Proven

Over 6.5 million specimens across approximately 750 sites and laboratories have been tracked in GlobalTrace, with 100% accountability of all specimens maintained.
Latest Resources
Fact Sheet
GlobalTrace
Case Study

GlobalTrace supports
National Study of
SARS-CoV-2 Vaccines
Whitepaper

GlobalTrace: Do you know
where your Biospecimens are?